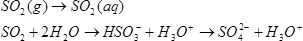
Throughout its history the diesel engine has always maintained its status as the most efficient system for
converting fuel into mechanical energy, and this situation is expected to continue for the foreseeable future.
The systems for after treatment focus on:
- The reduction of NOₓ
- The reduction of SOₓ
- The reduction of particulates (soot and particulate matter)
In order to achieve the lowest emission possible an after treatment of the exhaust gases is imperative. For the
reduction of sulphur, nitrogen oxides and particulates a variety of promising options is available. These options
have to be evaluated and the right combination of technologies has to be chosen for each application.
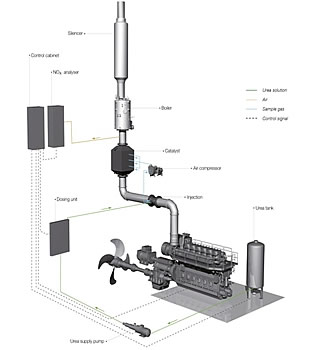 Figure 1 - SCR system
Figure 1 - SCR system
NOₓ storage catalytic converter
In the usual operation of a diesel engine a global excess of air is present in the combustion chamber. In this
mode high NOₓ emissions are present. With a NOₓ storage catalytic convertor the NO oxidizes on a Platinum surface to
NO2 and is embedded in the converter due to a reaction of the NOₓ with the contained barium carbonate which is
forming Ba(NO3)2.
The gaseous NOₓ is stored on the substrate surface and has to be regenerated from time to time. The NSC has a
reduction potential of up to 90% and works without a secondary consumable. Detrimental is the regular change between
storage and regeneration. This leads to a higher fuel oil consumption and requires changes in the engine
infrastructure. The biggest disadvantage is that the NOₓ storage catalytic convertor requires a fuel with very low
sulphur content because the Sulphur reacts with the barium carbonate to barium sulphate thus inhibiting the storage
reaction and thereby poisoning the converter.
Selective catalytic reduction
With a selective catalytic reduction catalyst the nitrogen oxides are reduced by use of ammonia. Ammonia
reacts with the oxygen out of the NOₓ and reduces those to nitrogen and water. The SCR catalyst has a high reduction
potential (over 90%) without influencing the engine operation (except for temperature control at certain
conditions). The SCR will only have a small influence on the fuel consumption (due to the increased back pressure
and temperature control).
The SOₓ emissions of the engine can not be influenced by the combustion process in the engine. The most
commonly used scrubber types in the marine industry are dry- and wet scrubbers.
Dry scrubber
In the dry scrubber the desulphurization process for the exhaust gas are based on absorptive processes. The
sulphates in the exhaust gas are reacting with calcium hydroxide to form calcium sulphate also known as gypsum.
This way the SOₓ is separated from the exhaust stream and stored in a chemical bond with calcium, thus forming
solid gypsum which can be stored on board of the ship and processed on land to usable product.
In the right configuration a dry scrubber possesses a significant SOₓ reduction potential while only
generating a minor impact on exhaust gas back pressure and temperature.
Wet scrubber
In the wet scrubber the desulphurization takes place in two steps, first the SOₓ is absorbed by the water.
In a second step the sulphur compounds are neutralized. This neutralization depends on the used wet scrubbing
mode. Either the neutralization is done by seawater (open loop) or by a fresh water caustic soda mixture (closed
loop).
In both cases a high sulphur reduction rate is possible. In the open loop sea water is pumped into the
scrubber and the absorption of the SOₓ takes places, as a second step the sulphur compounds are neutralized
primarily due to the carbonate system in the water. In the closed loop the absorption of the SOₓ is done with a
water caustic soda mixture. The neutralization is done primarily by the caustic soda.
This soda/water mixture is recirculated and cooled by sea water. From time to time a small amount of bleed off
water is taken out of the circulation tank and subsequently cleaned. The cleaned water is than either discharged
directly or stored in a holding tank. The washed out components (like soot, unburned hydro carbons) are stored in a
sludge tank.
The closed loop has the ability to cope with every water condition and discharges no polluted water. So this
system can be run permanently. On the downside it needs an auxiliary material; caustic soda, which is not easy to
handle.
The aim of the particulate filter is to reduce the emission of particles from the exhaust gas system. The
particles in the exhaust gas consist of soot (Carbon, also called black carbon) and particulate matter (these are
residual incombustible matter).
Two types of filters are most commonly used, a partial flow filter and a wall flow filter. Both systems have to
be regenerated from time to time. Aim of the regeneration is the oxidation of the soot (mainly the carbon) thus
decreasing the backpressure of the system.
For this process two different methods are possible: active regeneration and passive regeneration. For the
active regeneration the temperature has to be increased to the ignition point. For the passive regeneration the
activation energy is decreased and the regeneration takes place at lower temperatures.
Partial flow filter
The partial flow filter is an open system. In this type the exhaust gas stream is guided into filter bags and
the soot particles accumulate along the filter walls. However the exhaust gas stream is not forced to pass through
the walls. When the bags are filled the exhaust stream can go through the axial canal. This way the filter back
pressure is limited. But due to the open structure a certain amount of the exhaust gas can flow through the filter
without being cleaned, thus limiting the efficiency at around 80%.
Wall flow filter
The wall flow filter is a closed system. In this design the exhaust gas has to go through the filter walls.
During the passage through the wall a big part of the soot agglomerates on and in the filter wall. First the small
particles are going inside the filter wall and getting caught in small pores, while the bigger soot particles form a
filter cake on top of the wall. This filter cake builds up over time and increases the efficiency of the whole
system, because the exhaust gas has to pass the filter cake + the filter wall. While the build up of the filter cake
increases the efficiency, it also leads to a higher back pressure, which is limited by the engine.